√完了しました! pk assay principle 993104-What is the principle of mtt assay
Biological assays Yossi Levy 2 What is a bioassay?Assay Principle The amount of free ATP added to the reaction mixture which is consumed during the kinase reaction can be accurately measured using the patented bioluminescent Luciferase kinase reagents from Lonza A stable light signal is emitted that has an intensity which is proportional to the concentration of ATP presentGyrolab assays can be developed in a variety of matrices, including serum or plasma, sputum, CSF and cell culture supernatant Furthermore, you have full flexibility in choosing an assay format – such as standard sandwich, indirect and bridging Develop assays using your own reagents or use offtheshelf kits from Gyros Protein Technologies

Pharmacodynamics Wikipedia
What is the principle of mtt assay
What is the principle of mtt assay- PK assay bioanalytical testing methods are used to determine concentration time profiles of the drug and metabolites in biological sample fluids, providing information necessary for PK analysis PK assays are a vital component of the drug development process, and the data derived is used to help select dosage for preclinical and clinical studies Whilst, the specific details of such assays are beyond the scope of this article, it is important to emphasize that the basic principles of assay development must be adhered to in order to build robust and reliable assays that generate meaningful data Commercial assay development kits are available to ease the assay establishment




Tion Decreased To 6 87 Assuming The Mo Assay Chegg Com
HIV 1/2 StatPak For use with whole blood, serum, or plasma Store Kits 8 30° C • Check kit before use Use only items that have not expired or been damaged • Bring kit and previously stored specimens to room temperature prior to use • Always use universal safety precautions when handling specimens Keep work areas clean and organized Invented by Paul K Smith of the Pierce Chemical Company in 1985, it is also called the Smith Assay The assay determines protein concentration based on the colour change of the test sample from a pale green to an incredibly awesome purple, with the intensity of the purple being directly proportional to the amount of protein in the sample140 10 units DNAPK, 150 μM ATP 02μg/μl DNAPK peptide substrate, 60 min IC 50 = 1501 nM Wortmannin, nM % Enzyme Activity Figure 3 DNA‐PK Kinase Assay Development (A) DNA‐PK enzyme was titrated using 150µM ATP and the luminescence signal generated from each of the amounts of the enzyme is shown
Which ATPase assay is better Malachite green assay or LDH/PK coupled assay for my protein of interest?178 Adherence to the principles presented in this guideline will improve the quality and consistency of 179 the bioanalytical data in support of the development and market approval of both chemical and 180 biological drugs 181 The objective of the validation of a bioanalytical assay is to demonstrate that it is suitable for itsWatch also the principle of Bradford Assyhttps//wwwyoutubecom/watch?v=hdb3s4YHkms
The assay should be validated and assay acceptance criteria should be set in a fitforpurpose manner and DO NOT need to meet ALL of the same standards as a PK assay However, every effort should be made to evaluate critical assay parameters (eg standard curve performance, stability, specificity, parallelism, etc)From a free PK assay may be more informative for one project, while data from a total assay may be more informative for another project Going into a new biotherapeutic development program, we do not typically know a priori if there is likely to be a difference between data from a free versus a total biotherapeutic drug assay This is becauseMesoScale Discovery (MSD) Assays Measure a range of analytes in complex samples matrices consistently and at high sensitivity with our MSD assay capabilities Applications include ADA, NAb, and TAb assays, PK assays, and more




Pyruvate Kinase Assay Kit Ab432 Abcam



Establish Drug Pk Analysis Method Based On Competitive Elisa
For a biologic therapeutic, Pharmacokinetics (PK) and Immunogenicity are two important measurements in support of safety and efficacy While PK measure the drug in the system, Immunogenicity qualitatively determines the binding and the neutralizing antibody presence induced by the drug Below are two examples of how AIT Bioscience has used the oneassay approach first applied to a PK and immunogenicity method for one biosimilar, and the second applied to a nonclinical immunogenicity method for a different drug program Case 1 PK and Immunogenicity in a Standalone Study AIT Bioscience used a oneassay approach to developThe PK CMVPA System has been developed to provide an indirect particle agglutination CMV assay using uniform reagents, which are stable and easy to handle Automation has enhanced the value of the indirect particle agglutination test by significantly reducing the amount of time and labor needed to perform the assay




Adp Glo A Bioluminescent And Homogeneous Adp Monitoring Assay For Kinases Assay And Drug Development Technologies
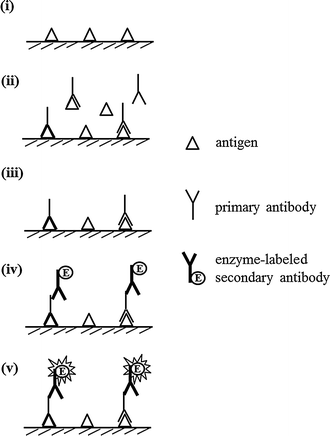



Enzyme Linked Immunosorbent Assay For The Quantitative Qualitative Analysis Of Plant Secondary Metabolites Springerlink
Pyruvate Kinase Activity Assay Kit Colorimetric & Fluorometric Assay for Measuring Pyruvate Kinase Activity in various Biological Samples such as Blood, Tissues, Cultured cells etc within 30 min Convenient, Accurate & Reliable Detection Limit 01 mUProtocols are available for each type of PK assay and ADA assay for many of the antibiotherapeutic products in our catalog PK and ADA Bridging ELISA Protocols Bridging ELISA is a special case of a sandwich ELISA in which a dimeric or oligomeric antigen (most often an antibody in a sample) is detected by a capture and detection antibodyIn fact, the drug interference or under detecting of ADA in all three cases was eliminated This assay principle could be used not only for ADA assays but also PK and biomarker (drug target) analysis in the presence of interference factors




Enhancing Value Of Clinical Pharmacodynamics In Oncology Drug Development An Alliance Between Quantitative Pharmacology And Translational Science Venkatakrishnan 17 Clinical Pharmacology Amp Therapeutics Wiley Online Library




Hit To Lead And Lead To Candidate Optimisation Using Multi Parametric Principles Drug Discovery World Ddw
PK PD Analysis Services PK PD analysis study examines a drug's effect compared to its exposure in the body after dosing PK PD assays estimate the safety and efficacy of therapeutics after suitable bioanalysis Pharmacokinetics modeling and simulation help further understand what the body does to a drug, modeling the processes of absorption BIOLOGICAL PRINCIPLES OF THE TEST The Chembio HIV 1/2 STATPAK™ Assay should be stored in its unopened pouch at 8 to 30°C (46 to 86°F) Do not freezeIn molecular biology Proteinase K (EC 3, protease K, endopeptidase K, Tritirachium alkaline proteinase, Tritirachium album serine proteinase, Tritirachium album proteinase K) is a broadspectrum serine protease The enzyme was discovered in 1974 in extracts of the fungus Engyodontium album (formerly Tritirachium album) Proteinase K is able to digest hair (), hence,
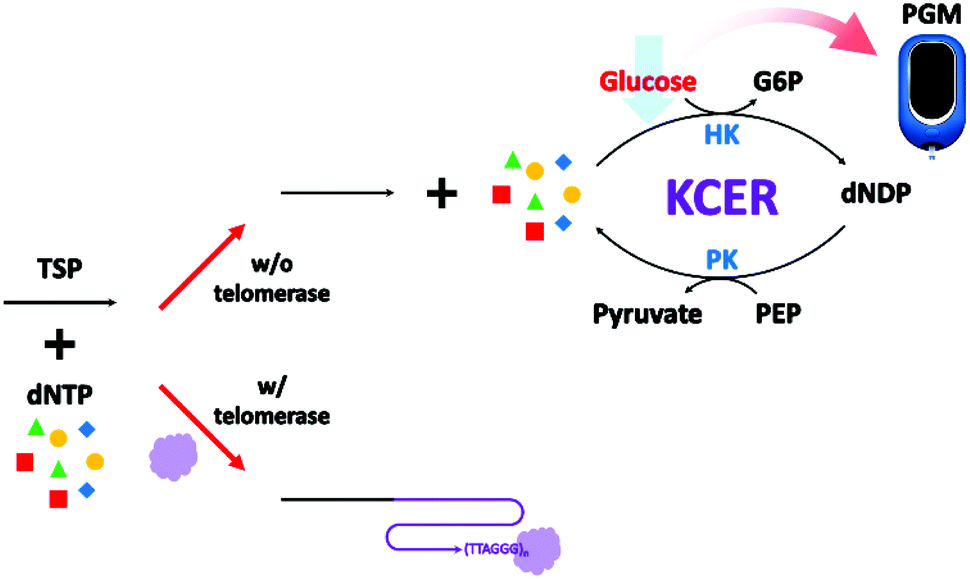



Portable Glucose Meter Utilized Label Free And Washing Free Telomerase Assay Analyst Rsc Publishing
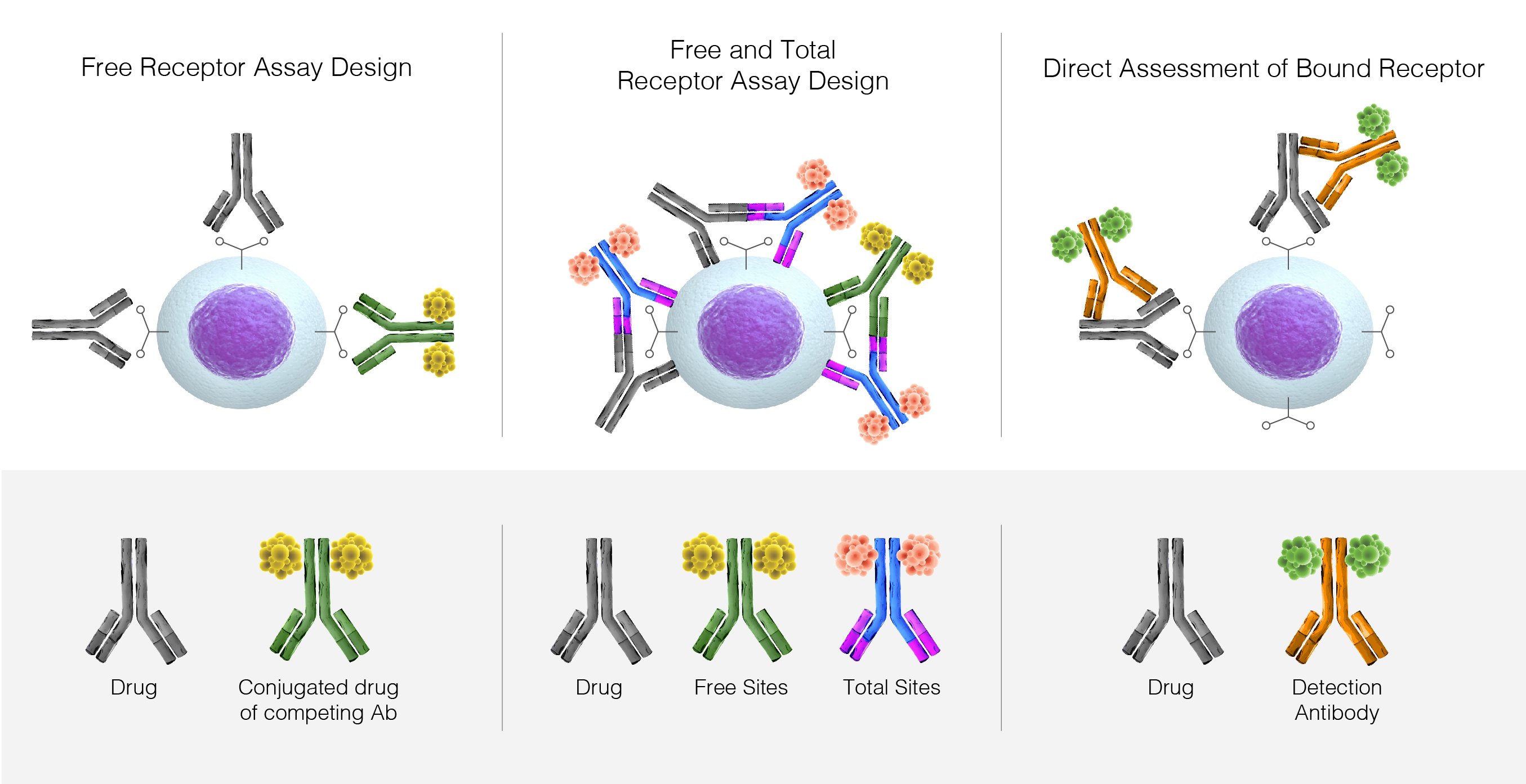



Receptor Occupancy Definition Overview Applications
Pharmacokinetics (from Ancient Greek pharmakon "drug" and kinetikos "moving, putting in motion";A typical suite of assays required during biotherapeutic development includes drug concentration assay (pharmacokinetic or PK assay) and assays detecting presence of antidrug immune response Multiple assays may be required to determine appropriate compound PK as well as to characterize an antidrug immune response Pyruvate Kinase Assay Kit ab432 provides a simple, direct and automationready assay for measuring pyruvate kinase activity in cell culture supernatant, plasma, serum, tissue, urine and other biological fluids (not validated for use with whole blood) In the pyruvate kinase assay protocol, PEP and ADP are catalyzed by pyruvate kinase to




Pharmacodynamics Wikipedia




Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis
Biological assays The MSD product line includes a diverse menu of single and multiplex assay kits for profiling biomarkers, cell signaling pathways, and other applications, as well as a suite of plates and reagents for assay development MESO SCALE DISCOVERY A division of Meso Scale Diagnostics, LLC 1601 Research Blvd Rockville, MD 850 USAEnzymes are biological catalysts (also known as biocatalysts) that speed up biochemical reactions in living organisms, and which can be extracted from cells and then used to catalyse a wide range of commercially important processes This chapter covers the basic principles of enzymology, such as classification, structure, kinetics and inhibition, and also provides an overview of industrialPfizer Confidential Time hrs 0 100 0 300 400 500 600 700 MaAb ug 1 10 Free and Partially Bound (Target Capture) Total



Resources Perkinelmer Com Lab Solutions Resources Docs App Alphalisa Pharmacokinetic Tnfa Pdf



1
Bioassays are for estimating the potency of a drug by utilizing the reaction caused by its application to live experimental subjects Bioassay always compares a test substance to a standard substance Assumptions Comparable organisms Same active compound Only concentration can varyMicrobial assays or microbiological assays could be a sort of bioassays designed to analyse the compounds or substances that have impact on microorganisms They help to estimate concentration and efficiency of antibiotics 19 What is difference between purity and assay?Antibody based PK assay was used to quantify concentrations of rituximab and ocrelizumab (unpublished data) These results prompted investigations of the human PK assay formats and reagents used in each assay to understand the PK differences observed The assay format used for rituximab quantification was a polyclonal antiCDR (PAC) assay, and for




Tion Decreased To 6 87 Assuming The Mo Assay Chegg Com




Smc Immunogenicity Testing Assay Life Science Research Merck
See chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determine the fate of substances administered to a living organism The substances of interest include any chemical xenobiotic such as pharmaceutical drugs, pesticides, food additives,Pyruvate Kinase Assay Method The reaction velocity is determined in a lactate dehydrogenase coupled assay system by measuring the decrease in absorbance at 340 nm resulting from the oxidation of NADH One unit of activity causes the oxidation of one micromole of NADH per minute at 25°C and pH 76 under the specified conditions ReagentsPK and TK Gyrolab ® immunoassays at nanoliterscale reduce sample volume requirements and animal use Pharmacokinetic (PK) and toxicokinetic (TK) bioanalysis presents challenges at all stages of development for the safety and efficacy of biologics Early in development, there is a need to support multiple therapeutic candidates and multiple



Www Mesoscale Com Media Files Technical notes Assay development plates uncoated Pdf




Pdf Drugability Studies Are Keys To The Successful Commercialization Of Biotherapeutics Semantic Scholar
Meso Scale Discovery Assay is a bioanalysis platform that utilizes electrochemiluminescence, unlike the colorimetric or chemiluminescent reaction in ELISA, as a signal detection technique MSD ECL Assays are superior to traditional ELISA in many aspects, even as the various viable assay versions are quite like different types of ELISADiluted PK positive control is measured with the PK Assay kit after various reaction times (Figure 2) The linear range of detection is 03 to 96 U/ml in a 96well plate assayTypical PK assays in ½ Area 96well plate B) AlphaPlex assay Protocol for PK assay in 384well OptiPlate A B Figure 4 Adalimumab antibody comparison A) A very sharp Hook can be seen using the high affinity (K D =006 nM) antiAdalimumab antibody (HC04) on both sides of the




Pyruvate Kinase Lactic Dehydrogenase Enzymes From Rabbit Muscle For The Determination Of Adp Buffered Aqueous Glycerol Solution Sigma Aldrich




Alphalisa Immunoassay Kits
Assay Validation & Performance Reports Biologics only • Immunogenicity • Comparability Clinical Pharmacology • FirstinHuman • SAD and MAD PK Studies • Healthy vs Patient populationPK Assay Buffer 25 mL OxiRed™ Probe 02 mL PK Enzyme Mix (Lyophilized) 1 vial PK Substrate Mix (Lyophilized) 1 vial PK Positive Control (~18 mU, Lyophilized) 1 vial Pyruvate Standard (100 nmol/µl) 100 µL Store kit at °C OXIRED™ PROBE Ready to use as supplied Allow to come to room temperature before use to melt frozen DMSOPK Assay Impact of lack of confidence in data Target Ligand ged Anti human IgG Drug Free and Partially Bound (human IgG) Enlightened PK/ PD Driven Bioanalytical support for IVT study Assay format defines form of analyte?




Gyrolab Assay Protocol Automated Immunoassays Gyros Protein Technologies
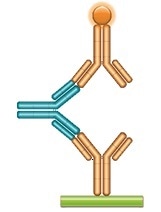



Using A Bridging Assay For Anti Drug Antibody Detection
The main difference between assay and purity is that an assay is theTo improve assay sensitivity, neither approach was widely applicable for routine drug analysis The desire to apply PK principles to drug discovery would not only require an improvement in assay sensitivity, significant increases in analytical capacity andOr ADA on the PK assay need to be considered in the design of the sampling strategy by looking for active or total drug Chirmule et al, AAPS J 14(2), (12) Ø The success of the PK–PD modeling effort depends on close communication between the PK–PD bioanalytical and clinical scientists The PK–PD model is
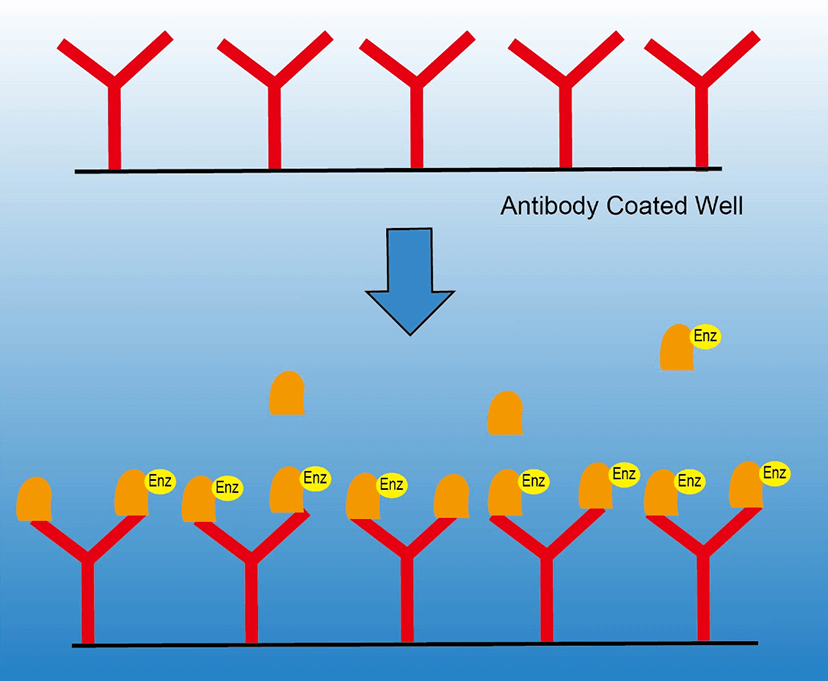



Competitive Elisa Protocol Creative Diagnostics



Http Microbiology Ucdavis Edu Heyer Wordpress Wp Content Uploads 13 11 Atpase Assay Pdf
Quanterix ® SIMOA™ Assays For clinical development programs that need biomarker detection with high sensitivity and broad dynamic range, Precision uses the Quanterix SIMOA platform The SIMOA SRX platform can deliver 1000fold higher sensitivity than traditional ELISAs, yielding analyte detection in the femtomolar (fM) range and enablingPyruvate kinase (PK) is an enzyme that catalyzes the final step of glycolysis, Principle The Pyruvate Kinase Activity Assay Kit provides a simple and direct procedure for measuring pyruvate kinase activity in a variety of biological samples Pharmacokinetic (PK) measurements are critical in all phases of antibody therapeutic (AbT) development, starting from target/molecule selection during the discovery stage, PK projections from animal to human to inform doses for early clinical studies, to safety/efficacy evaluation during proofofconcept and confirmatory clinical studies (Phase II/III)




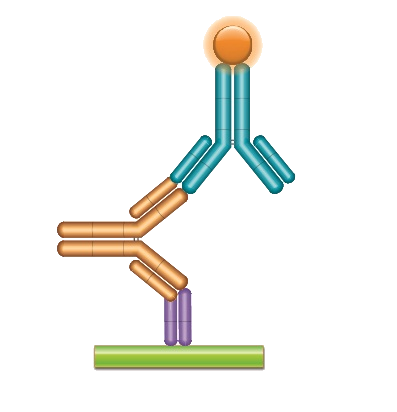
Schematic Presentation Of The Assay Principle For Human Igg Download Scientific Diagram




Pharmacokinetics Wikipedia
Immunoassays are used to quantify molecules of biological interest based on the specificity and selectivity of antibody reagents generated In HTS and lead optimization projects, assays are designed to detect molecules that are produced intracellularly or secreted in response to compounds screened This chapter describes the basics of designing and implementingHello, I have a protein whose kd value for ATPase activity is pretty low ( uM) French essai "trial", and the noun assay thus means "trial, test of quality, test of character" (analytic) in laboratory medicine, pharmacology, environmental biology, and molecular biology Specificity ability of the test/ assay to detect true negatives Repeatability Same observation using same instruments and operators, and over short time periods Reproducibility




Pharmacokinetic Pk Assays Pk Pd Analysis Dmpk Assay




Pyruvate Kinase Assay Kit Ab432 Abcam




Chemoprobe Based Assays Of Histone Lysine Demethylase 1a Target Occupation Enable In Vivo Pharmacokinetics And Pharmacodynamics Studies Of Kdm1a Inhibitors Journal Of Biological Chemistry




On Site Therapeutic Drug Monitoring Trends In Biotechnology
.png.aspx)



Assays For Covid 19 Drug Discovery
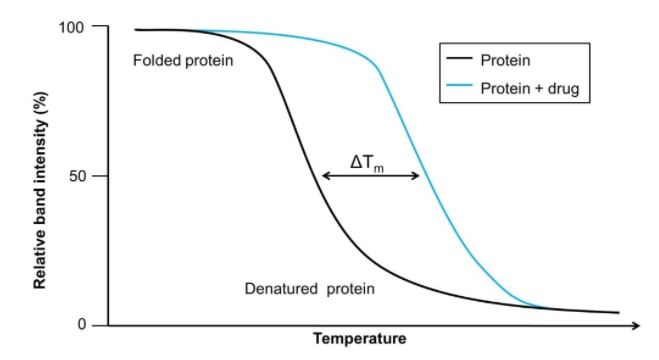



Cellular Thermal Shift Assay Cetsa Platform Sygnature Discovery




In Vitro And In Vivo Assessment Of Adme And Pk Properties During Lead Selection And Lead Optimization Guidelines Benchmarks And Rules Of Thumb Assay Guidance Manual Ncbi Bookshelf
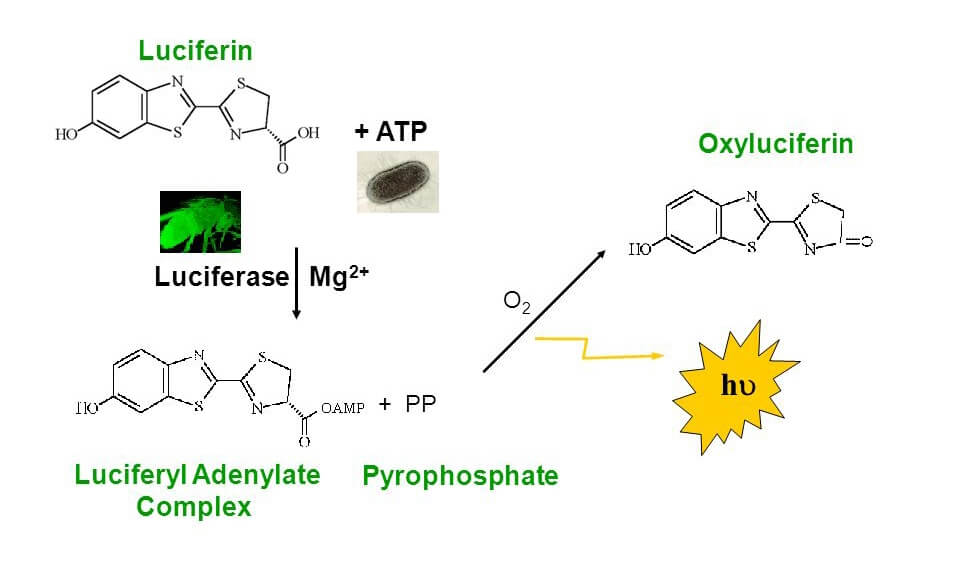



Atp Cell Viability Assay Creative Bioarray



Q Tbn And9gcsatmt 5kt0 Wkkxd7lx9hwkdqbb4 Qe Ah8ufgdumh7jvqtsyt Usqp Cau
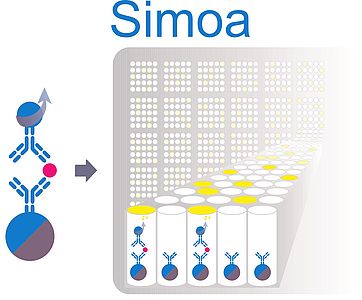



Simoa Immunoassays Services Chimera Biotec Gmbh




Pharmacokinetics Wikipedia




Pyruvate Kinase Assay Kit Ab432 Abcam
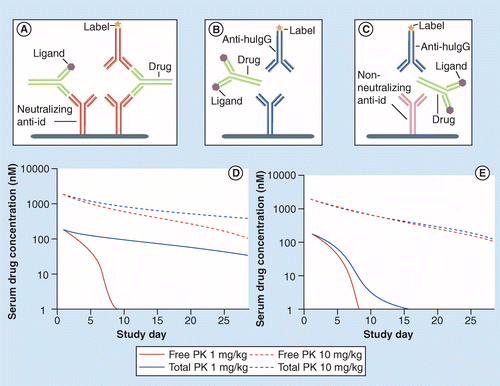



Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis




Pk Tk Gyrolab Automated Immunoassays Gyros Protein Technologies




Immunoassay Methods Assay Guidance Manual Ncbi Bookshelf




Pharmacokinetics For Haemophilia Treaters Meaning Of Pk Parameters Interpretation Pitfalls And Use In The Clinic Thrombosis Research
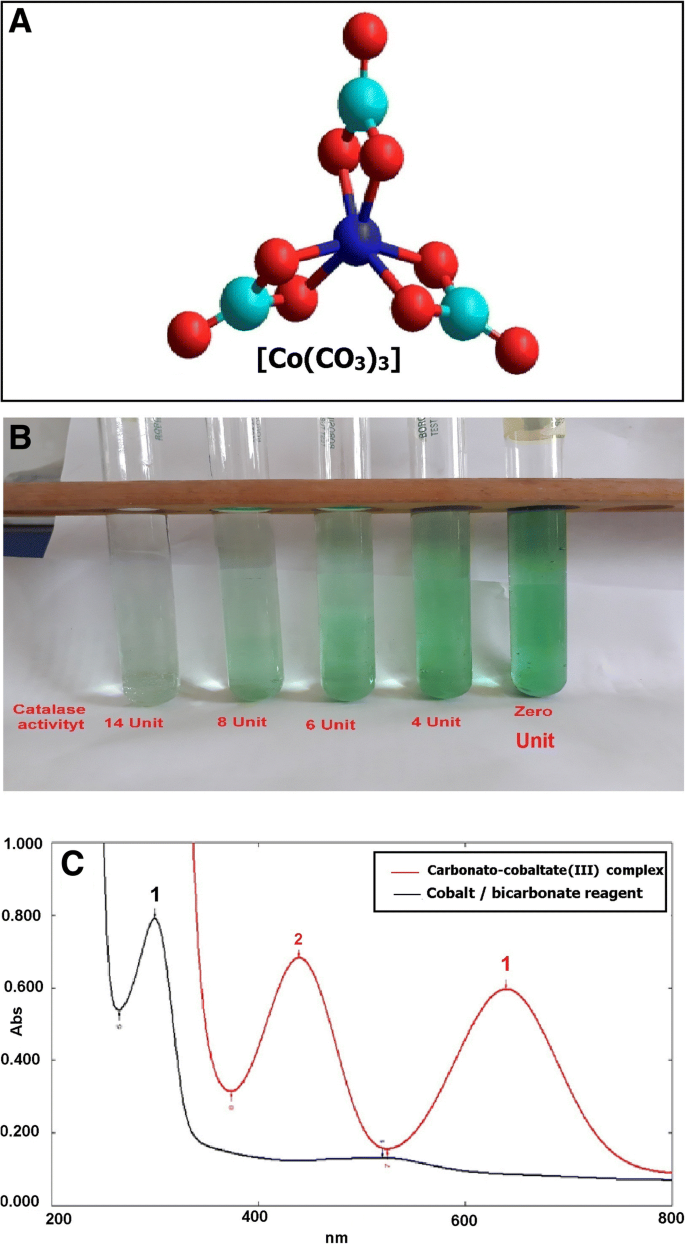



Simple Spectrophotometric Assay For Measuring Catalase Activity In Biological Tissues Bmc Biochemistry Full Text
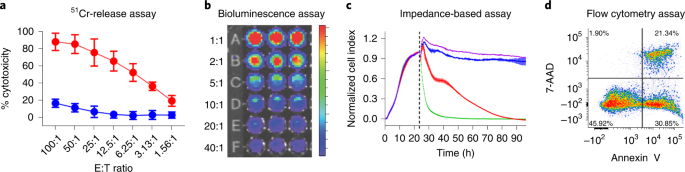



Comparative Analysis Of Assays To Measure Car T Cell Mediated Cytotoxicity Nature Protocols
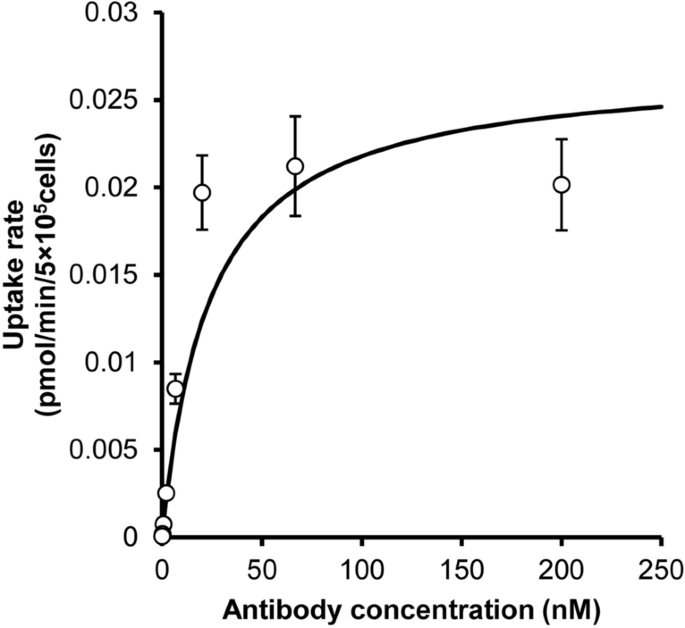



Pharmacokinetic Prediction Of An Antibody In Mice Based On An In Vitro Cell Based Approach Using Target Receptor Expressing Cells Scientific Reports
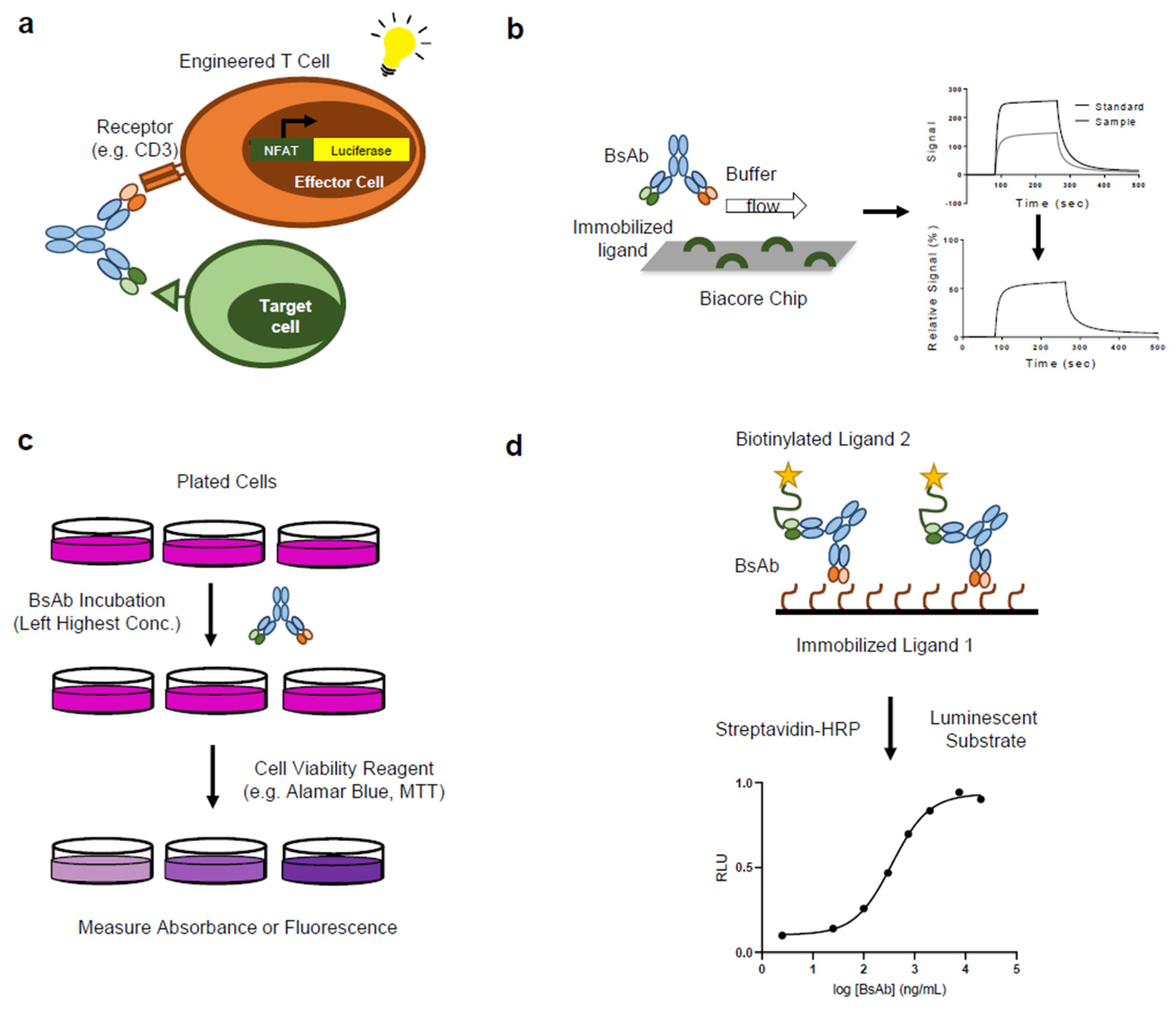



Ijms Free Full Text Bioassay Development For Bispecific Antibodies Challenges And Opportunities Html



1
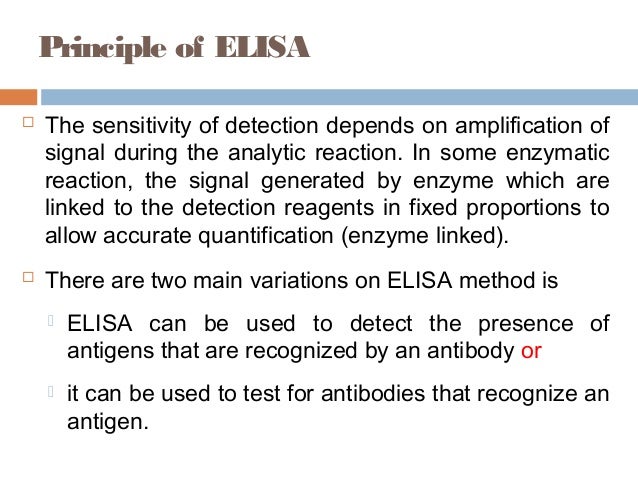



Principles And Applications Of Elisa




Principle Of The 2 Site Immunometric Typing Assay In The Screening Download Scientific Diagram
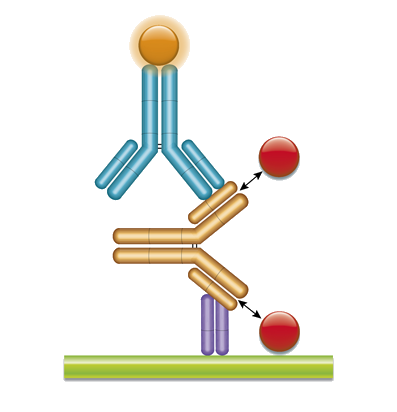



Elisa Protocols Bio Rad
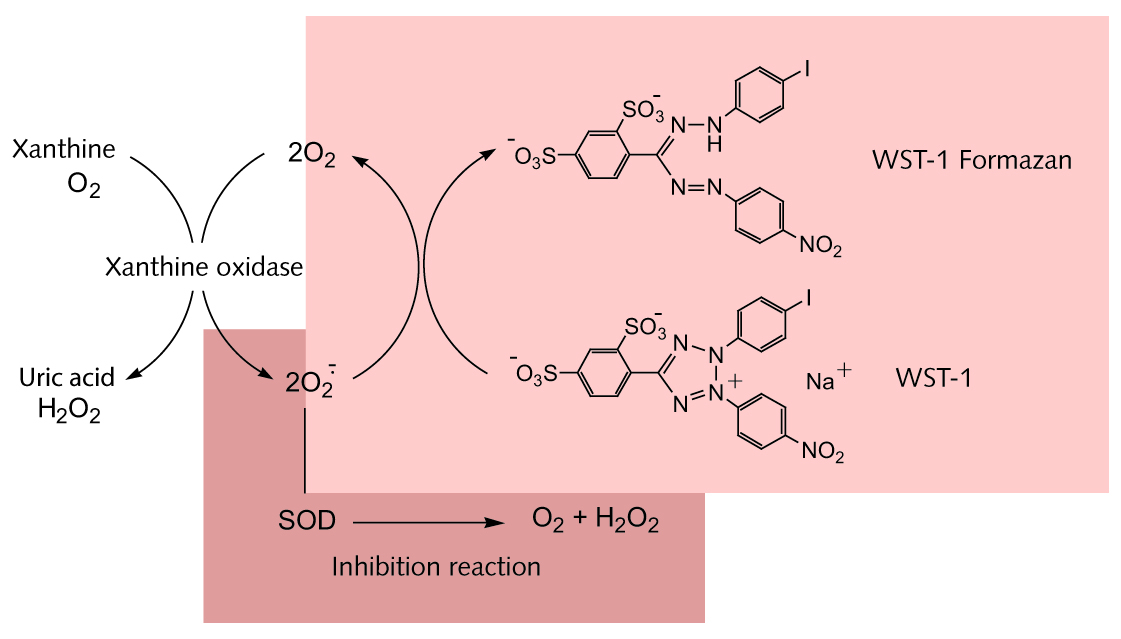



Sod Assay Kit Promocell




Scielo Brazil Non Clinical Studies In The Process Of New Drug Development Part Ii Good Laboratory Practice Metabolism Pharmacokinetics Safety And Dose Translation To Clinical Studies Non Clinical Studies In The




Dtxswdhnpjevhm




High Throughput Elisa Ready To Analyse In 90 Minutes 384 Well Simplestep Elisa With The Pherastar Fsx




B Arrestin Recruitment Assays
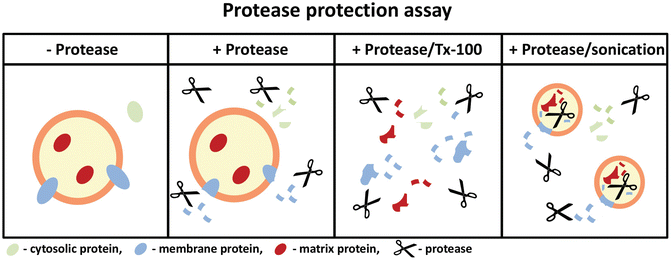



Determining The Topology Of Peroxisomal Proteins Using Protease Protection Assays Springerlink




Principle Of The Proteinase K Assay Cryosections Are Incubated With Pk Download Scientific Diagram
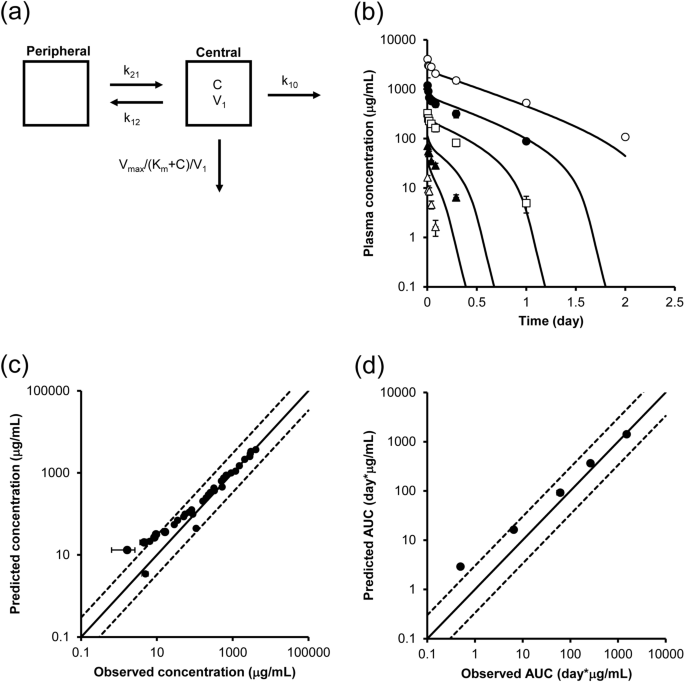



Pharmacokinetic Prediction Of An Antibody In Mice Based On An In Vitro Cell Based Approach Using Target Receptor Expressing Cells Scientific Reports




Pharmacokinetic Studies In Children Recommendations For Practice And Research Archives Of Disease In Childhood




Pyruvate Kinase Assay Kit Ab432 Abcam




Pharmacokinetic Assay An Overview Sciencedirect Topics




Pharmacokinetic Assay An Overview Sciencedirect Topics



1




Elisa Euroimmun Ag




Acid Dissociation To Detect Ada And Drug 1 Illustration Of A Sample Download Scientific Diagram



Research Utexas Edu Wp Content Uploads Sites 6 16 08 Drug Discovery Development Process 2 Pdf




Cytosmart Wound Healing Assay What Why And How




Assay Techniques And Test Development For Covid 19 Diagnosis Acs Central Science



Establish Drug Pk Analysis Method Based On Competitive Elisa
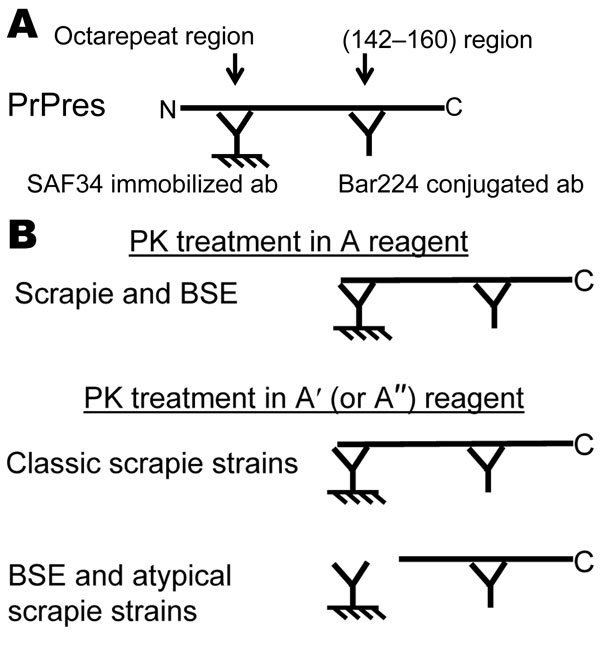



Figure 1 Rapid Typing Of Transmissible Spongiform Encephalopathy Strains With Differential Elisa Volume 14 Number 4 April 08 Emerging Infectious Diseases Journal Cdc




Indirect Assessment Of Neutralizing Anti Drug Antibodies Utilizing Pharmacokinetic Assay Data Sciencedirect



Http Tools Thermofisher Com Content Sfs Brochures Tr0065 Elisa Guide Pdf
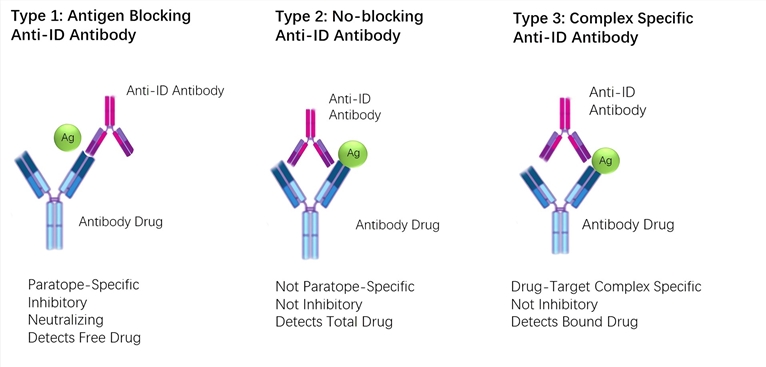



Pharmacokinetic Pk Antigen Capture Elisa Measuring Bound Drug Exclusively Creative Biolabs




Protein Translocation



The Difference Between Pharmacokinetics And Pharmacodynamics Bioagilytix
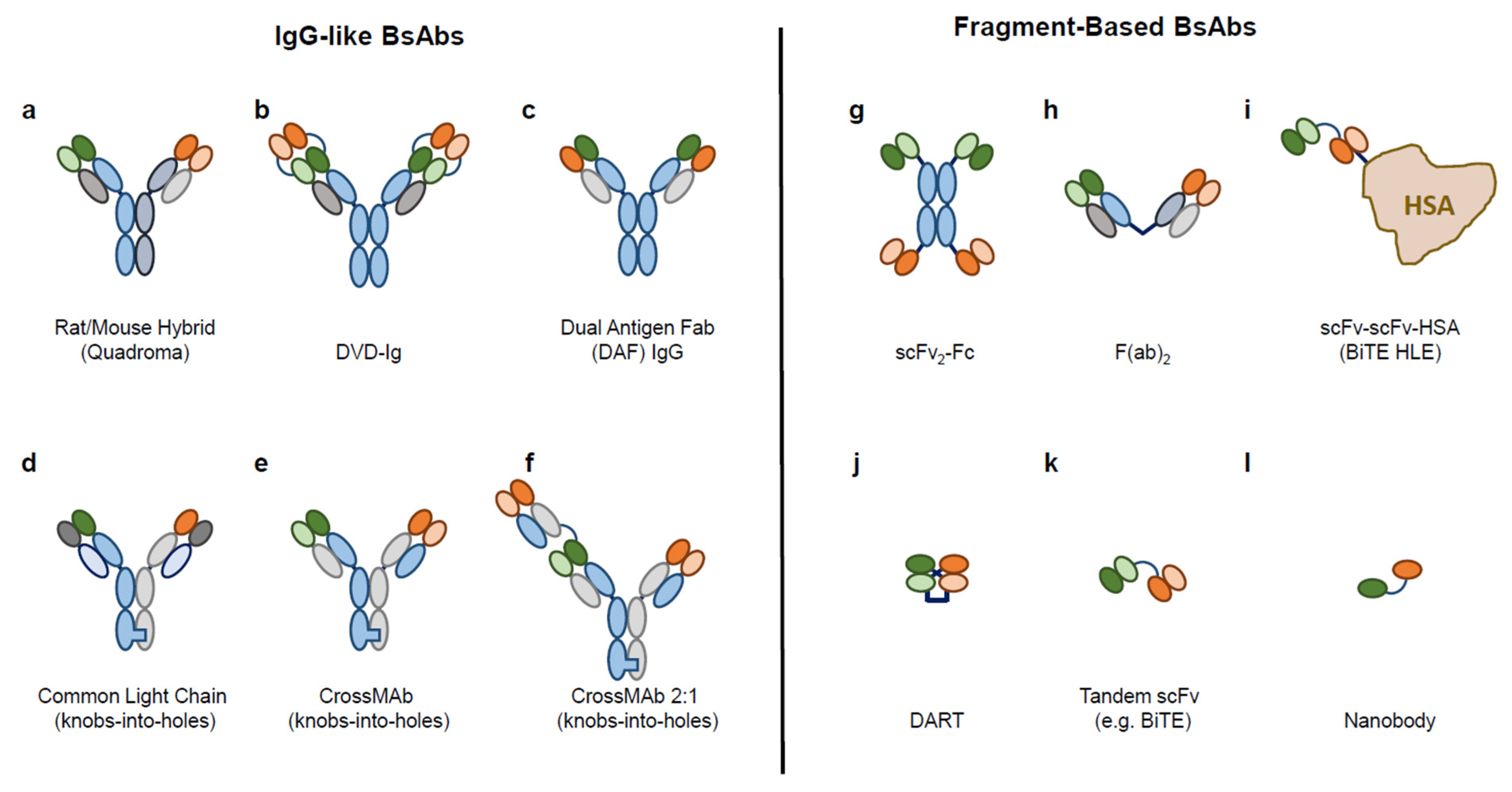



Ijms Free Full Text Bioassay Development For Bispecific Antibodies Challenges And Opportunities Html




Pharmacochaperone Assays Discoverx




Receptor Binding Assay An Overview Sciencedirect Topics




Herg Safety Assay




Quantitation Of Lysosomal Trapping Of Basic Lipophilic Compounds Using In Vitro Assays And In Silico Predictions Based On The Determination Of The Full Ph Profile Of The Endo Lysosomal System In Rat Hepatocytes



Pharmacodynamics Wikipedia




Simple How Do Anti Drug Antibody Assays Work Youtube




Mechanistic Projection Of First In Human Dose For Bispecific Immunomodulatory P Cadherin Lp Dart An Integrated Pk Pd Modeling Approach Chen 16 Clinical Pharmacology Amp Therapeutics Wiley Online Library




Immunogenicity Testing Of Therapeutic Antibodies In Ocular Fluids After Intravitreal Injection Bioanalysis



Www Ashp Org Media Store files P2418 Sample Chapter 1 Pdf




Package Insert Labs Inc



Elisa Protocols Bio Rad
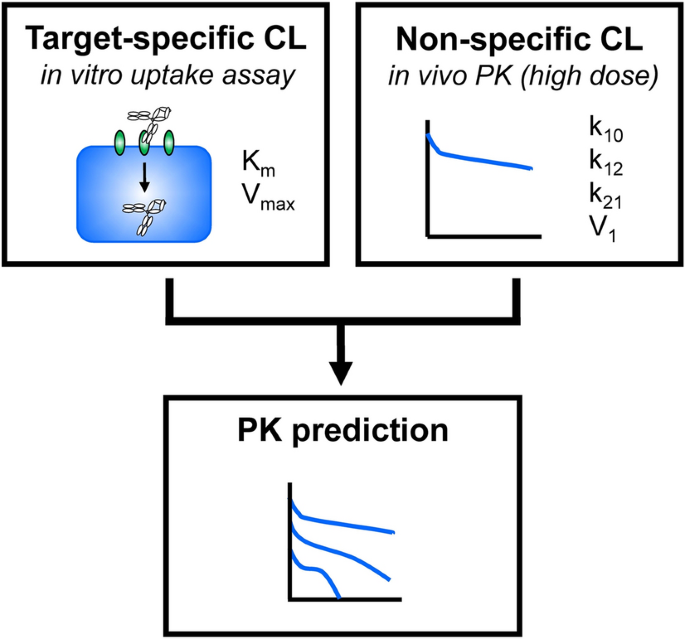



Pharmacokinetic Prediction Of An Antibody In Mice Based On An In Vitro Cell Based Approach Using Target Receptor Expressing Cells Scientific Reports




Schematic Presentation Of The Assay Principle For Human Igg Download Scientific Diagram
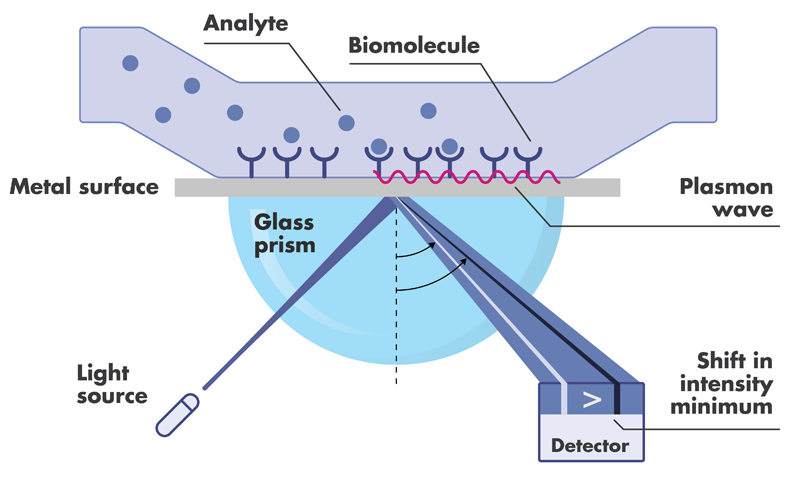



Spr Biacore Assay Services Reaction Biology




Mesoscale Discovery Msd Assays Precision For Medicine



Receptor Dimerization Assays



Resources Perkinelmer Com Lab Solutions Resources Docs App Alphalisa Pharmacokinetic Tnfa Pdf
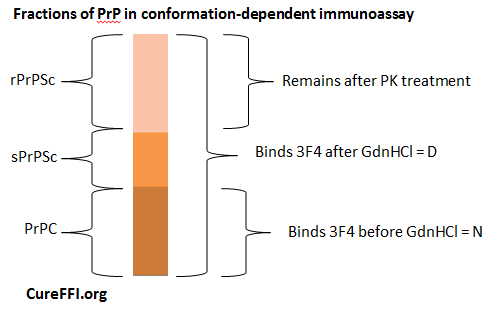



Introduction To Conformation Dependent Immunoassay




Receptor Binding Assay An Overview Sciencedirect Topics




Pk Assay Analysis Services Pharmacokinetics Pk Study Pk Testing Northeast Biolab
.png.aspx)



Interleukin Cell Based Assays




Biophysical Properties Of The Clinical Stage Antibody Landscape Pnas
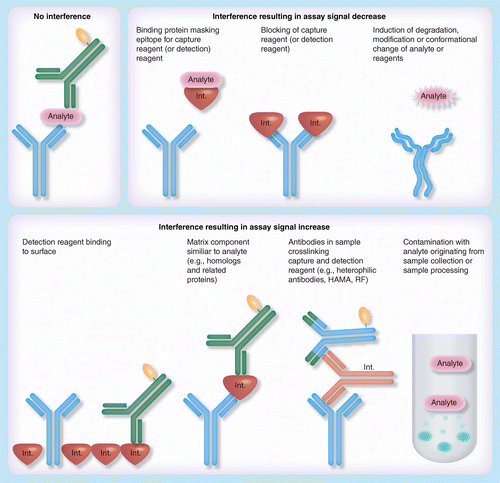



Interference In Immunoassays To Support Therapeutic Antibody Development In Preclinical And Clinical Studies Bioanalysis




Novel Drug And Soluble Target Tolerant Antidrug Antibody Assay For Therapeutic Antibodies Bearing The P329g Mutation Bioanalysis
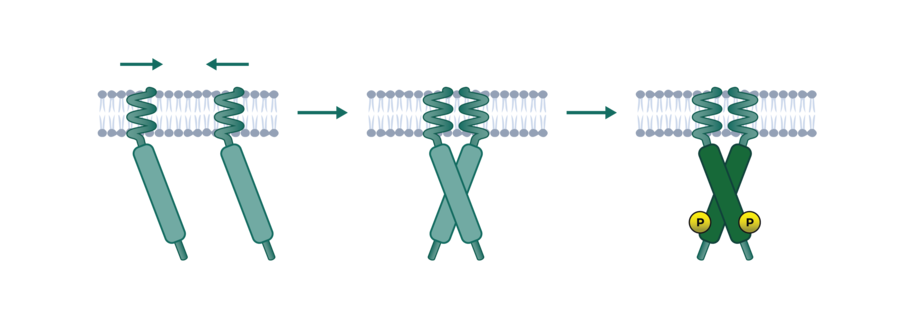



Cellular Phosphorylation Assay Service Reaction Biology




Use Of Ella To Facilitate Drug Quantification And Antidrug Antibody Detection In Preclinical Studies Bioanalysis
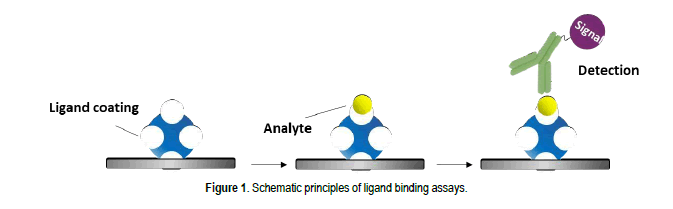



A Review On Lba And Lc Ms Platforms For Supporting Large Molecule Pharmacokinetics Bioanalysis




Application Of Pk Pd Modeling In Veterinary Field Dose Optimization And Drug Resistance Prediction




Meso Scale Discovery Msd Pacific Biolabs




Pharmacokinetic Assay An Overview Sciencedirect Topics



コメント
コメントを投稿